Antibiotic resistance is rising to dangerously high levels in all parts of the world. New resistance mechanisms are emerging and spreading globally, threatening our ability to treat common infectious diseases. A growing list of infections – such as pneumonia, tuberculosis, blood poisoning, gonorrhea, and foodborne diseases – are becoming harder, and sometimes impossible, to treat as antibiotics become less effective. Without urgent action, we are heading for a post-antibiotic era, in which common infections and minor injuries can once again kill.


Learn more about how Armata is leading the fight against antibiotic resistance

Antibiotic resistance will remain a major threat to public health unless alternative antimicrobial therapies are developed.
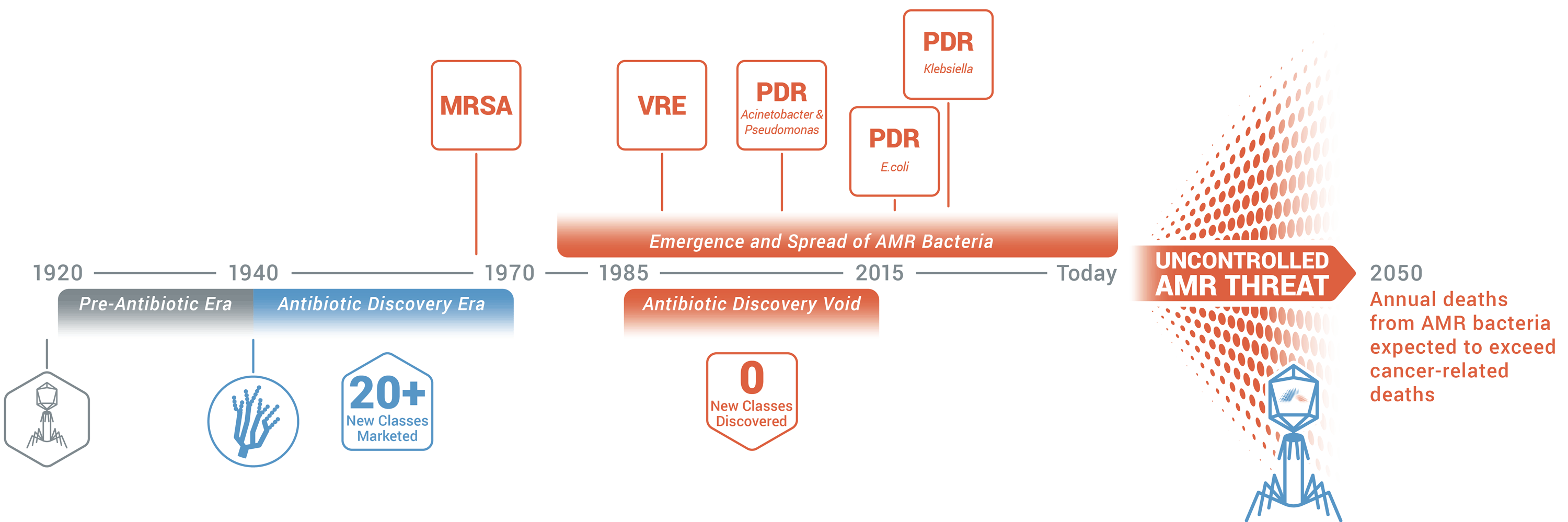
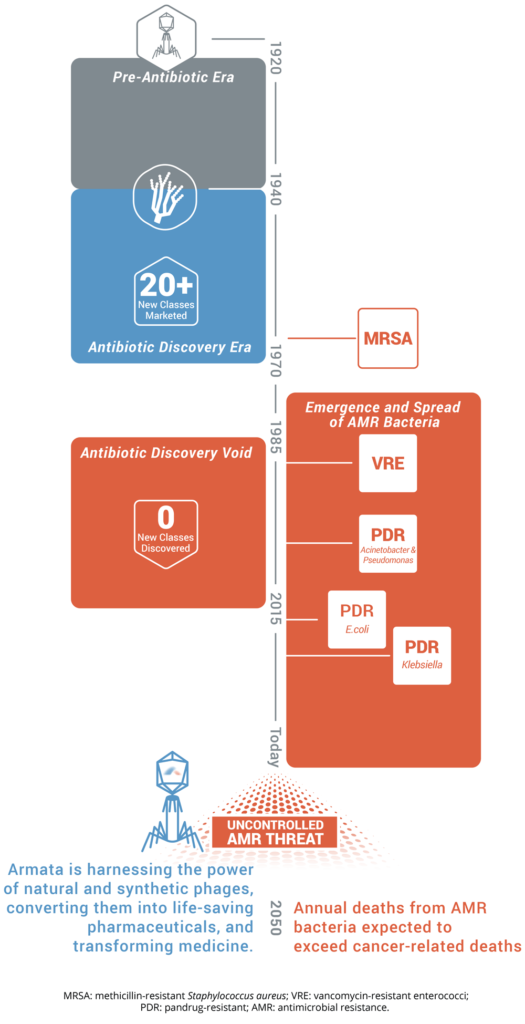
Armata is harnessing the power of natural and synthetic phages, converting them into life-saving pharmaceuticals, and transforming medicine.
MRSA: methicillin-resistant Staphylococcus aureus; VRE: vancomycin-resistant enterococci; PDR: pandrug-resistant; AMR: antimicrobial resistance.

See what scientists worldwide are saying about antibiotic resistance
A spike in deadly infections has forced scientists to find new ways to fight superbugs. CNN chief medical correspondent Dr. Sanjay Gupta spoke with researchers who are looking to nature in the fight against them. 04:28 – Source: CNN. Click box below to view the video.
Learn more about the Antimicrobial Resistance crisis here »


